There are 4 general categories of biohazardous wastes based on the physical form of the waste. Each form must be segregated, identified, decontaminated and disposed of in an appropriate manner for the form in order to minimize occupational exposure and environmental release risks.
Biohazardous waste in any form should not be left unsecured in areas that are accessible to the public (i.e., left in hallways). Only lab personnel should remove biohazardous waste from the lab area and transport it to waste holding areas for final disposal.
In the research lab or field environment, this includes any non-sharp item that is contaminated with human or animal diagnostic specimen material (i.e., body fluids, tissue debris), any microbiological culture material (including recombinant DNA).
Examples include but are not limited to:
- Gloves and other disposable PPE contaminated with specimen or culture material
- Plasticware such as pipettes or pipette tips, culture plates, specimen vials, etc. that are contaminated with biological specimens, bacterial and cell culture material, or nucleic acids
- Towels and bench paper that are biologically contaminated (Note: Bench paper that is used in areas where samples or cultures are opened and manipulated must be regarded as biologically-contaminated and therefore removed and managed as solid biohazardous waste)
- All culture or sample containers that are contaminated with biological materials
- Tubes of blood (note: glass blood vials that could break easily upon disposal should be segregated as sharps waste; see below)
Storage
 Non-sharps solid biohazardous waste must be collected for final treatment and disposal in a leak-proof container lined with an autoclavable bag of moderate thickness to prevent punctures. The collection container must have a lid or other means of closure and the container must be labeled with the biohazard symbol regardless of the lab’s operating biosafety level. For BSL-2 labs, bags must be red, orange, or embossed with the biohazard symbol.
Non-sharps solid biohazardous waste must be collected for final treatment and disposal in a leak-proof container lined with an autoclavable bag of moderate thickness to prevent punctures. The collection container must have a lid or other means of closure and the container must be labeled with the biohazard symbol regardless of the lab’s operating biosafety level. For BSL-2 labs, bags must be red, orange, or embossed with the biohazard symbol.
Benchtop containers should be used for the collection of small quantities of contaminated dry goods (i.e., pipette tips, centrifuge tubes, etc.). Small plastic containers or wire bag racks lined with a biohazard bag are suitable for benchtop collection. These containers do not need to have a lid (unless waste is contaminated with a pathogen) but daily disposal of the secured bag into a larger collection container such as the one shown to the right is strongly recommended.
“Breakable” biohazardous wastes
Tubes of blood and other “breakable” biohazardous waste can be troublesome to manage properly and safely for treatment and disposal. For small amounts of “breakable” biohazardous waste, these items may be placed in sharps containers for disposal. However, if your lab generates a large amount of “breakable” biohazardous waste, please contact the UTK/UTIA Biosafety Officer for assistance with finding solutions for safer waste management.
Serological Pipettes
Pipette wastes may require creative approaches for accumulation prior to disposal. Serological pipettes and micropipette tips are good examples, as they may not fit some biohazardous waste bins or may present a sharps hazard if they comingle with heavier wastes (e.g., agar plates).
- Line a cardboard box, 5-gallon bucket, or similar container with a biohazard bag and collect the pipettes with the tips oriented in the same direction.
- Ensure the outside of the container has a biohazard label.
- When the bag is full, treat the pipettes by autoclave or disposal through the medial waste contractor as indicated above.
- Pipette tips may be placed in the sharps container or other puncture-resistant collection devices.
Pathological Waste
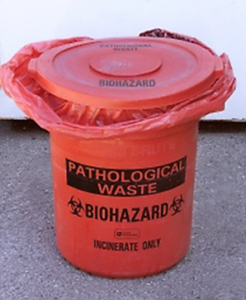 Animal tissues, tissue trimmings, organs, or carcasses must be:
Animal tissues, tissue trimmings, organs, or carcasses must be:
- Collected in leak-proof, sealed bags. Bags must be red, orange, or embossed with the universal biohazard symbol if the pathological material contains an infectious agent, recombinant/synthetic nucleic acid, or biological toxin.
- Frozen and stored (tissues) for disposal through the respective lab animal facility or satellite facility.
- Unless otherwise indicated by EH&S Laboratory Safety Services, pathological waste is not to be autoclaved. Rather it will be shipped offsite for incineration or submitted to the College of Veterinary Medicine’s necropsy unit for alkaline hydrolysis.
NOTE: Never discard pathological wastes into the trash!
The collection of autoclaved soil, plants, and plant materials must be conducted in a manner that follows the correct waste stream and does not create ergonomic risks for facilities personnel. This guidance applies to the collection, treatment, and disposal of all soil and plant material as defined below. This shall apply to all students, staff, and faculty responsible for handling and disposing of soil, plants, and plant materials.
Guidance
Soil and plant wastes do not qualify as ‘regulated medical waste’ per state code, so these are not subject to special segregation into the medical waste contractor receptacles. However, to meet this exemption these wastes cannot be collected in an autoclave bag that is red (or orange) or bears the biohazard symbol (i.e., it must be a plain white/clear/tan autoclave bag). The bags can then be placed directly into the dumpster once autoclaved/cooled. Dry cycles can be incorporated into soil sterilization to possibly help reduce the weight of autoclaved soil. It is important to follow this guidance for autoclaved soils and plant materials as they contribute 11x more to the cost of disposal than other materials in the waste stream. This guidance also applies to USDA permitted soils and plant materials as long as they are fully devitalized prior to final disposition.
Field Generation
Waste should be collected and stored as previously outlined. Contact the UTK/UTIA Biosafety Officer or UTIA Safety Officer for assistance with identifying disposal options. If you plan to transport waste for treatment and/or disposal, please refer to guidelines at the end of this document for this activity.
This includes bulk quantities of blood, blood products, body fluids from human and animal research origin and culture media. Note: Disposable primary containers or sample containers containing small quantities of liquids (less than 10 mls) should be managed as solid biohazardous waste.
Storage
 These liquids must be stored in closed, leakproof containers while awaiting treatment and disposal. Collection vessels should be secured so that they cannot be tipped over. Secondary containment is strongly recommended and can be achieved by placing the vessel in a bucket or deep tray. Storage vessels or the secondary container must be labeled with the biohazard label if the liquids will not be treated and disposed of within the shift. If a disinfectant is added to the vessel, provide labeling so that the chemical hazard is identified as well. For instance, if your collection flask contains waste cell media and bleach, place a biohazard label on the flask (or secondary container) as well as the words “bleach-treated cell culture materials” to properly identify both the chemical and biological hazard.
These liquids must be stored in closed, leakproof containers while awaiting treatment and disposal. Collection vessels should be secured so that they cannot be tipped over. Secondary containment is strongly recommended and can be achieved by placing the vessel in a bucket or deep tray. Storage vessels or the secondary container must be labeled with the biohazard label if the liquids will not be treated and disposed of within the shift. If a disinfectant is added to the vessel, provide labeling so that the chemical hazard is identified as well. For instance, if your collection flask contains waste cell media and bleach, place a biohazard label on the flask (or secondary container) as well as the words “bleach-treated cell culture materials” to properly identify both the chemical and biological hazard.
Treatment and disposal
Liquid wastes may be treated and disposed of by either one or the other of the following methods:
- Chemical treatment of liquids with disinfectant; disposal via lab sink
Disinfectants may be used for “treatment” of liquid biological waste to prohibit the growth of microorganisms. Here is an example of the use of household bleach. Add household bleach to the collection vessel so that the bleach makes 10% to 15% of the final volume. Allow a contact time of at least 30 minutes. Carefully discharge the mixture to the sanitary sewer by way of the lab sink, then thoroughly rinse down the sink with water. Remember to wear splash goggles, gloves, and a lab coat for the handling of bleach and bleach-treated liquids.
NOTE: Diluted bleach solutions may go down the drain in most cases. However, many chemicals used for disinfection cannot be discarded down the train. Contact EH&S at 974-5084 to determine if sink disposal of disinfectants other than diluted bleach solutions is acceptable.
- Autoclave treatment of liquids; disposal via lab sink
Place the closed collection vessel in a secondary container and transport it by cart to the autoclave facilities. Treat by autoclave using the liquids cycle. (Remember to loosen or remove the closure on the vessel before placing in the autoclave.) Discharge cooled treated liquids to the sanitary sewer by way of the lab sink. Note: Only personnel who have received training regarding the operation of the autoclave should use this device.
Safety Note: PLEASE do not autoclave liquids containing chemical disinfectants!
Biohazardous sharps waste must be disposed of in an FDA-approved container that is manufactured for the disposal of biohazardous sharps waste: 1) puncture resistant; 2) restricted opening disallowing retrieval of sharps; 3) a lid that can be securely closed once full; 4) labeled with the universal biohazard symbol.
- All sharps containers must be permanently closed and disposed of when 2/3 to ¾ full or whenever items do not freely fall into the container. Never pack, tamp, or shake a sharps container to fit additional items.
- Wipe down the exterior surface of the container with a disinfectant prior to submission for disposal.
- Clean sharps may also be placed in the red sharps containers as necessary.
Disposal for non-sharps and sharps biohazardous waste
Basic procedure:
-
- Collect biowastes at the laboratory level in designated biohazard-labeled cans per current biowaste collection procedures. Segregate serological pipettes and pipette tips to prevent bag punctures or tears. Collect biohazardous sharps in sharps containers as required (see below).
- The following are acceptable wastes: stock/propagated cultures of infectious agents; materials that have been used for the collection/processing/storage of human or animal blood or body fluids (including cell lines); recombinant/synthetic nucleic acids; or lab consumables contaminated with any of these materials.
- The following are unacceptable wastes: hazardous chemicals (e.g. phenol, chloroform, agarose gels with ethidium bromide, etc.); radioactive wastes; bulk liquid wastes (>25 ml/container); pathological waste such as human/animal bulk blood, tissues, or animal carcasses (contact Biosafety for guidance); human fetal remains, limbs, or cadavers; compressed gas cylinders; loose sharps; controlled substances; etc.
- Once the bag is ~2/3 full, close the bag by gathering the top, twisting, and closing with a single overhand knot. This method of closure minimizes the risk of leaks and spills and is required by DOT (since materials will be transported in commerce). Do not tie the bag closed by crossing tabs (‘bunny or dog-eared’ method).
- Collect biowastes at the laboratory level in designated biohazard-labeled cans per current biowaste collection procedures. Segregate serological pipettes and pipette tips to prevent bag punctures or tears. Collect biohazardous sharps in sharps containers as required (see below).
- Once the bag is properly closed, double bag it using the same closure technique listed above. The bag can then be deposited directly into a medical waste contractor-provided receptacle, a ~90-gallon bin emblazoned with the company’s name, the universal biohazard symbol, the UN shipping identifier (UN3291) and proper shipping name (Regulated Medical Waste, n.o.s.). Again, autoclaving bags prior to disposal will not be required, except for the following circumstances:
-
- Any infectious agent listed on the DOT Category A Infectious Substances list. For UT, this means that stock or propagated cultures of verotoxigenic E. coli, Shigella dysenteriae, and hepatitis B virus are to be autoclaved for a minimum of 30 minutes. Once treated, allow the bags to cool to room temperature, place in a second bag secured according to the above technique and then discard into a medical waste contractor-provided receptacle.
- Any material under federal permit (e.g. USDA APHIS) requiring onsite inactivation in an approved and validated autoclave. These must be autoclaved according to validated parameters. Once treated, allow the bags to cool to room temperature and then discard into a medical waste contractor-provided receptacle or dispose of in the dumpster. (Note: regulated plants/soils that require onsite inactivation should follow the procedure outlined at https://biosafety.utk.edu/biosafety-program/waste/.)
- Properly packaged and permanently closed sharps containers may also be discarded into the medical waste contractor receptacles. Sharps containers must be secondarily enclosed in a securely tied (as described above) biohazard bag prior to disposal. Permanently closed sharps containers must be wiped down with a disinfectant prior to removal from the lab for disposal. If there are any liquids present in the biohazardous sharps container, it must be placed in a leak-proof secondary container with a secure lid (and a biohazard label) for transport to the waste collection site or prior to bagging for depositing in the medical waste contractor bins. Disposal of biohazardous sharps containers will be accomplished through a medical waste disposal contractor coordinated through EH&S. Alternatively, sharps containers can be taken to the nearest hazardous chemical waste room (recommended if the sharps containers are very large). The collection of sharps containers in the waste collection rooms will be on the same schedule as chemical waste. Contact EHS for the current chemical waste collection schedule at 865-974-5084. Do not dispose of biohazardous sharps containers in the regular trash, regardless of treatment status.
- Be sure that all materials being placed into the medical waste contractor receptacles are double bagged prior to depositing packaged waste from the labs (including sharps containers). Lab wastes are not to be placed in the white ‘Autoclave-treated Wastes’ Brute® bins after the medical waste contractor receptacles are placed. Contact Facilities Services for pick up if white bins pictured below remain in your area after red bins have been delivered.
Autoclave validation program:
Once the Medical waste contractor process is in place, there is no longer a need to do quarterly autoclave validations to satisfy TDEC rules for landfilling treated biohazardous wastes. A subset of volunteers will be asked to continue quarterly validations with biological indicators per USDA permit requirements (as applicable). Autoclaves used to treat Category A Infectious Substances will also be tested at least annually. Otherwise, autoclave testing will be at the discretion of the respective users. EH&S Laboratory Safety Services will continue to supply Class 5 thermal integrator test strips and/or biological indicators upon request.
*Note on autoclaved plant and soil waste:
While these can technically be deposited into medical waste contractor bins once autoclaved, to reduce bin weight (and disposal cost) it is preferred that these are managed according to the SOP posted at https://biosafety.utk.edu/biosafety-program/waste/. Briefly, collect in clear/white/non-biohazard emblazoned bags, autoclave to tested parameters, allow to cool, and remove to the building dumpster.
Do not discard wastes not mentioned here into medical waste contractor containers. If waste is not described here, contact EH&S Laboratory Safety Services for further guidance.
On-site Autoclave Treatment (if required above)
Autoclavable waste bags must be used in biohazardous waste collection containers. Bags must be placed in a secondary container (i.e., a tray with raised sides), which is placed on a cart for movement to the autoclave facilities.
Practice notes on biohazard bags:
- Biohazard bags are a one-way means of disposal. Do not “dump” the contents from one biohazard bag into another as this action spreads contamination and increases your exposure to this waste.
- Biohazard bags need to be contained at all times during the collection, treatment, and disposal process. Some lab items may puncture bags and this can lead to leaks and spills. Bags awaiting autoclave treatment should be stored in trays, tubs, buckets, etc. (The only exception to this practice is when small quantities of biohazardous wastes that do not contain liquids are collected temporarily in benchtop containers.)
- Biohazard bags must not be used for the collection of other hazardous wastes (i.e., ethidium bromide gels).
Autoclave treatment of this waste must be performed in accordance with the biohazardous waste treatment parameters established for the autoclave. Note: Only personnel who have received training regarding the operation of the autoclave should use this device.





