EFFECTIVE SEPTEMBER 2022
Version 8/20/2024
IBC Registration, Reporting, Review, & Recordkeeping Procedures [PDF]
Authority for the Institutional Biosafety Committee (IBC) and its scope, purview, policies, and procedure are described in the IBC Charter & Bylaws document.
The IBC requires documentation of biological hazards used in research, teaching, and diagnostic testing, and associated procedures for their receipt, storage, use, and disposal. Principal investigators (PIs)/supervisors are expected to register and regularly update their biohazard inventory, handling procedures, laboratory infrastructure, and personnel. Documentation is reviewed by the IBC or the Biosafety Officer (BSO) and supporting staff (at the IBC’s discretion) and as allowable by regulatory standards. Registrations, amendments, updates, and supporting materials such as training records, material transfer agreements, equipment validations, regulatory permits, etc. will be maintained and managed by the BSO.
Registration
PIs must register research involving biological hazards through the University of Tennessee, Knoxville (UTK) web-based compliance management system, which is used for information collection, routing, and archiving. Information and instructions for the compliance management system is available through the UTK EHS-Biosafety website. Required information includes types of biological hazards used; applicable risk group(s) or NIH Guidelines review categories; non-technical and technical procedural summaries; laboratory location, biosafety level(s), and infrastructure; emergency procedures, occupational health requirements, and personnel training confirmation.
Respective information for teaching and diagnostic laboratories using biological hazards will be maintained by the BSO as per IBC discretion.
Federal or state-issued permits/authorizations (e.g., USDA [United States Department of Agriculture], APHIS [Animal and Plant Health Inspection Service], CDC [Centers for Disease Control]) for the receipt and use of biological hazards may be substituted for an IBC registration. Contingently, the permit/authorization must be provided to the BSO for review, compliance consultation, and recordkeeping. Copies of permits and associated documentation will be maintained by the BSO as per IBC discretion.
Reporting: Amendments & Updates
Amendments to registered biohazards (including recombinant or synthetic nucleic acid constructs), handling procedures, laboratory infrastructure, and personnel must be disclosed to the IBC so risk assessments and review/approval requirements can be determined in a timely fashion. In some cases, the IBC may require submission of a new registration (if research scope deviates from that in the approved registration) and/or preapproval (if amendment results in changes to the NIH Guidelines review category and/or biosafety level). Amendments to research registrations are made through the web-based compliance management system. Amendments to teaching, diagnostic testing labs, or those working under federal/state permits must be disclosed to the BSO for risk assessment, determination of IBC oversight, and documentation updates.
PIs are required to complete an annual update to active registrations. Annual updates capture project status, any changes to biohazards, procedures, facilities, or personnel, as well as any accidents/injuries/exposures that need to be disclosed to the IBC. Annual updates are made through the web-based compliance management system.
Failure to Register/Report:
Failure to register and/or willful neglect of reporting requirements is subject to the IBC’s escalation procedures. Administrative action for unresolved noncompliance may include the total shutdown of a lab or office, referral of the individual for disciplinary action, report to local, state, or federal regulatory and/or funding agencies and suspension of research.
Grace Period:
If needed, a grace period of 60 days (about 2 months) from the registration expiration will be given to execute committee business. The grace period is for committee business only and does not diminish the application or stringency of the IBC’s escalation procedures. The chair has the discretion to issue an extension letter past 60 days (about 2 months) for extenuating circumstances.
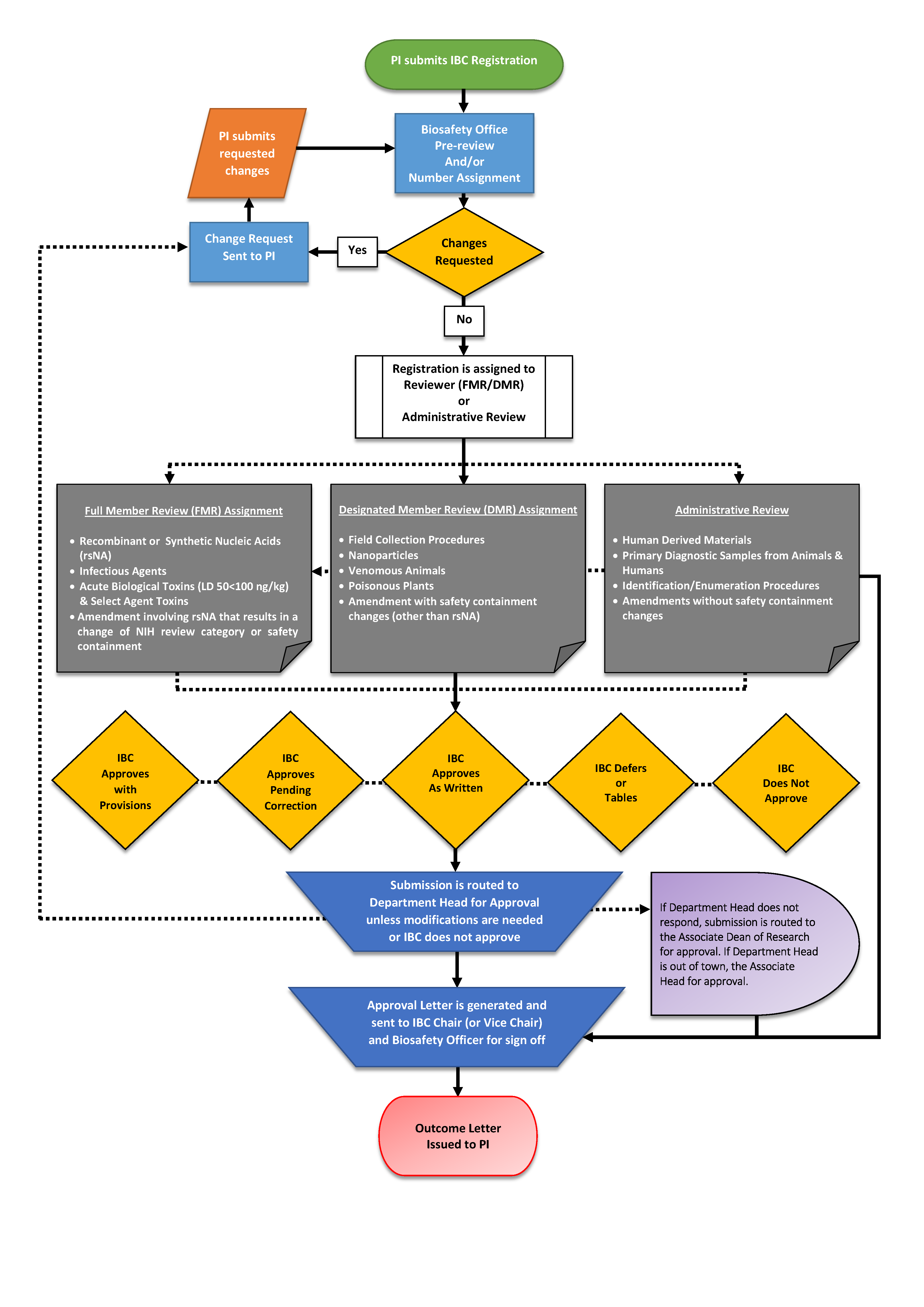
IBC registrations, amendments, and updates submitted through the compliance management system are routed and reviewed as follows:
- The BSO assigns a tracking number (for new registrations) and performs a pre-review to verify form completion, confirm the correct categorization of biological hazards and risk/biosafety level, and identify omissions or information inconsistencies.
- Revision requests are returned to the PI for completion.
- The BSO and/or IBC Chair assign the registration to full member review (FMR), designated member review (DMR), or administrative review (AR) depending on the following criteria:
- FMR requires review/approval by the full committee. FMR is required for the following:
- Initial registration of non-exempt recombinant and synthetic nucleic acids (rsNA)
- Initial registration of infectious agents affecting human health
- Initial registration of acute biological toxins (LD50 < 100 ng/kg) or select agent toxins
- Amendments/updates involving rsNA and resulting in a change of the NIH Guidelines review category
- DMR requires review by the IBC Chair (or Vice-Chair), BSO, and at least one voting committee member. DMR may be used for the following:
- Initial registration of field procedures involving biohazards (or potential exposures to biohazards)
- Initial registration of nanoparticles conjugated to biologically active or cell-modifying molecules
- Initial registration of venomous animals
- Initial registration of poisonous plants
- Amendments/updates that involve safety/containment changes
- FMR requires review/approval by the full committee. FMR is required for the following:
Materials reviewed by DMR are also distributed to the full committee for discretionary review. Any designated reviewer or voting committee member may call the submission to full committee review. Approvals granted by DMR will be disclosed to the full committee at the subsequent meeting.
- AR may be conducted by the BSO and/or the IBC Chair (Vice-Chair) at the discretion of the IBC. AR may be used for the following:
- Initial registration of exempt rsNA
- Initial registration of human-derived materials
- Initial registration of primary diagnostic samples from animals and humans
- Microbial identification/enumeration from diagnostic or environmental samples
- Amendments/updates that do not alter the NIH Guidelines review category (for rsNA) or involve safety/containment changes (conservative changes to vectors/inserts, personnel updates, equipment updates, etc.)
Submissions reviewed by AR may be called to full committee review by the BSO or IBC Chair (Vice-Chair) at discretion. Approvals granted by AR will be disclosed to the full committee at the subsequent meeting.
- Review results in one of the following decisions:
- Approved as written
- Approved pending minor corrections
- Approved with provisions/contingencies that must be addressed and reported back to the IBC
- Deferred (tabled) pending clarifications or additional information
- Not approved (unacceptable risk to health/environment, inadequate containment facilities, lack of training/expertise, etc.)
- Upon verification of revisions (as necessary), the registration is routed to the Department Head for approval*. If the Department Head remains unresponsive after three weekly reminders, the registration is routed to the Associate Dean of Research (ADR) for approval. (*If the Department Head is also the PI on the registration, it will automatically be routed to the ADR for approval.)
- Upon approval, the registrations/amendments/updates are routed to the IBC Chair (Vice-Chair) or BSO, as authorized for AR, for final verification.
Approvals are valid for three (3) years, contingent upon annual updates as described above.
A flow diagram of IBC review procedures is provided in Appendix A.
IBC records are maintained by the BSO. Records may be electronic or paper copies. Data management and security will follow the policies, procedures, and general oversight framework established by the University of Tennessee IBC. Records will be maintained for a minimum of three years post-expiration/termination or other documented transactions or events (e.g., training), whichever is most conservative.