APPROVED SEPTEMBER 2022
IBC Event Escalation Procedures [PDF]
Authority for the Institutional Biosafety Committee (IBC) and its scope, purview, policies, and procedure are described in the IBC Charter & Bylaws document.
To protect the campus community, the IBC is tasked with evaluating research, teaching, and diagnostic testing involving biological hazards and constantly monitoring safety and compliance. Should an individual (faculty, staff, student, etc.) commit or attempt to commit any inappropriate action as determined by the IBC or Biosafety Officer (BSO) and supporting staff, every effort will be taken to resolve issues with the involved individual(s) and laboratory leadership. However, refusal or failure to correct identified concerns will prompt the following escalation procedures (summarized in Appendix A).
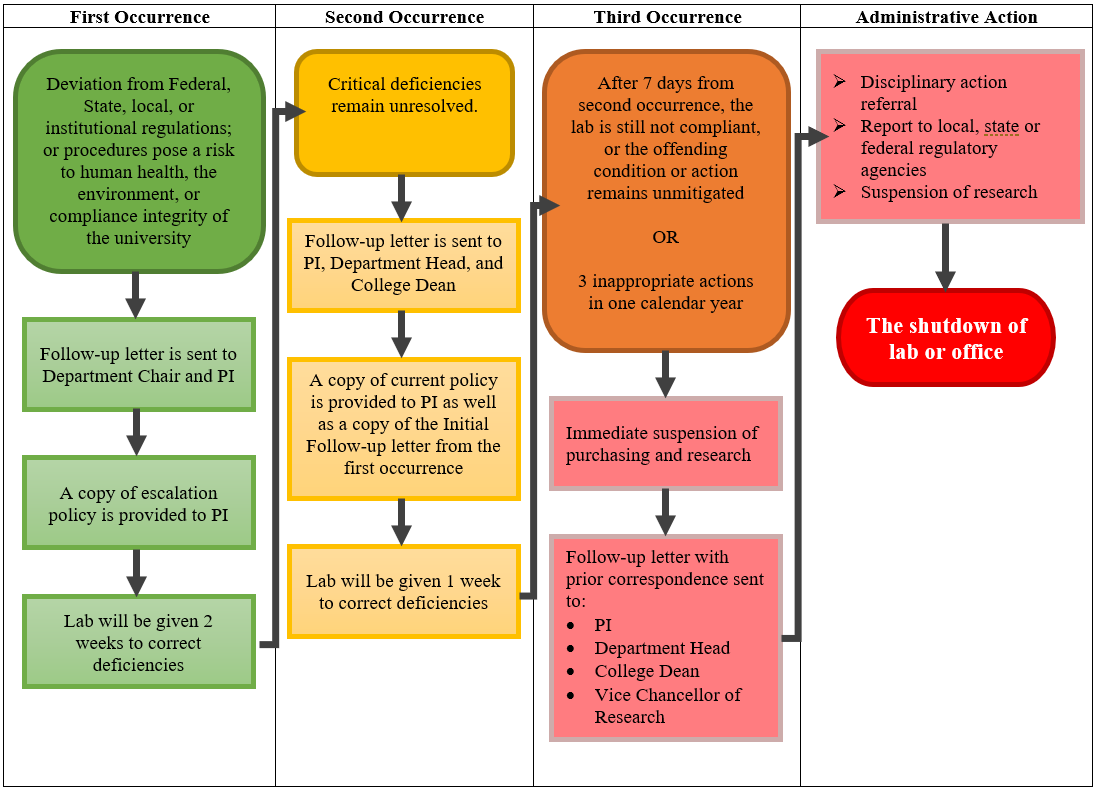
A first occurrence is constituted when a deviation from Federal, State, local, or institutional regulations, policies, or procedures poses a risk to human health, the environment, or the compliance integrity of the University of Tennessee; flagrant refusal to follow biosafety principles and practices; and/or the laboratory inspection response triggers the audit escalation procedure. A follow-up letter from the IBC Chair (or Vice-Chair) and BSO will be immediately sent to the principal investigator (PI)/laboratory supervisor and Department Head stating that inappropriate action has taken place and the consequences if such action continues. A copy of the current escalation policy will be provided.
Unless the deficiencies found are sufficiently critical to the life and health of the lab workers or the regulatory status of the laboratory, laboratories will be given a minimum of two weeks to correct deficiencies. Corrections will be verified by a follow-up audit.
If critical deficiencies remain unresolved, a follow-up letter from the IBC Chair (or Vice-Chair) and BSO will be immediately sent to the PI/laboratory supervisor, Department Head, and College (Associate) Dean stating that that inappropriate action has taken place and the consequences if such action continues and/or occurs again. A copy of the current escalation policy, as well as a copy of the initial follow-up letter from the first occurrence, will be provided.
Unless the deficiencies found are sufficiently critical to the life and health of the lab workers or the regulatory status of the laboratory, laboratories will be given one week to correct deficiencies. Corrections will be verified by a follow-up audit.
Purchasing and research will be suspended immediately if the following occurs:
- After seven days from the second occurrence, the lab is still not compliant, or the offending condition or action remains unmitigated, or
- The authorized individual or PI has committed three (3) documented inappropriate actions in any one calendar year.
A follow-up letter from the IBC Chair (or Vice-Chair) and BSO will be immediately sent to the PI/laboratory supervisor, Department Head, College (Associate) Dean, and Designated Official (DO) stating that the suspension has occurred with copies of all prior correspondence. Suspension of purchasing and research will continue until the Department Head, College (Associate) Dean, and DO approve reinstatement in cooperation with the IBC and respective research administration. It is at the discretion of the IBC to ultimately lift a suspension.
Diagnostic testing and teaching laboratories will follow a similar communication/escalation scheme; however, resolution is at the discretion of the respective department and/or college administration. Resolutions must be communicated to the IBC/Biosafety Office in a timely manner.
Serious actions include those that:
- pose an imminent threat to the life, health, or property of the University;
- are in violation of Federal, State, or local laws and may be subject to legal action;
- demonstrate (through documentation) an unwillingness to take corrective actions and/or repeated failure to follow programmatic and institutional policy; or
- defy the University Code of Conduct.
The IBC/BSO reserves the right to immediately stop work and/or shut down a laboratory for serious actions. To ensure prompt remediation, such orders may be issued irrespective of the escalation process and communicated in writing by the IBC Chair (or Vice-Chair) or BSO to any or all administrative levels.
Serious actions may also result in the suspension of research; total shutdown of a lab or office; notification of local, state, or federal regulatory and/or funding agencies (as required); or referral of the individual(s) for disciplinary action, as determined by University administration in cooperation with the IBC.
*The IBC/BSO reserve the right to issue an immediate stop-work order and/or laboratory shutdown for serious actions (as defined in the written procedure). To ensure prompt remediation, such orders may be issued irrespective of the escalation process and communicated in writing to any or all administrative levels.