What is a bloodborne pathogen (BBP)?
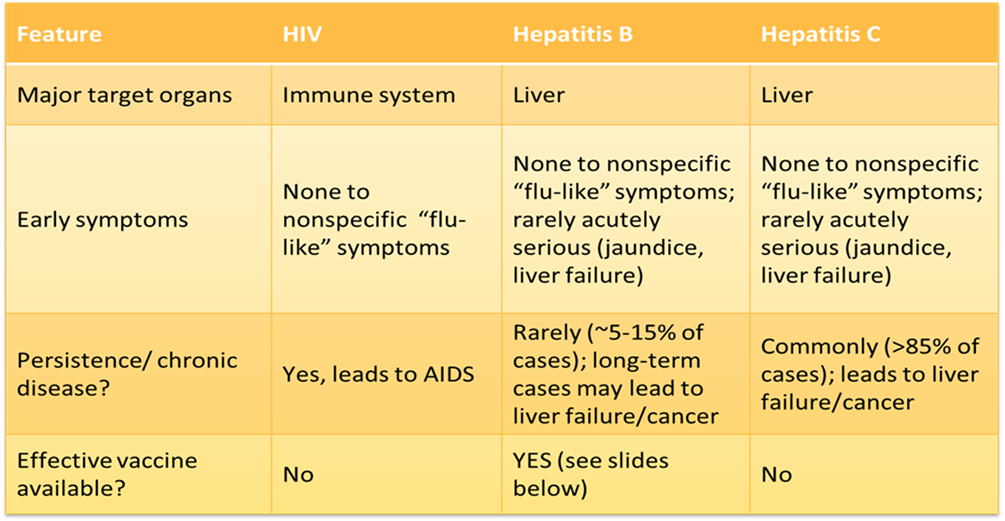
A BBP is a disease causing organism that can be found in human blood and certain body fluids. Common BBP include: Human immunodeficiency virus (HIV); hepatitis B Virus (HBV); hepatitis C virus (HCV). However, there are several other microbes that may be considered BBP, including:
- Viruses: HTLV-1; Epstein-Barr virus (infectious mononucleosis), Ebola virus, various arthropod-borne viruses (e.g. Zika)
- Parasites: Plasmodium falciparum (malaria)
- Bacteria: Treponema pallidum (syphilis)
The Occupational Safety and Health Administration’s (OSHA) Occupational Exposure to Bloodborne Pathogens (BBP) Standard [29CFR1910.1030] applies to anyone that will handle or may come into contact with human blood or other potentially infectious materials (OPIM) due to the risk of Bloodborne pathogens. OSHA mandates initial and annual recurrent training for all affected personnel.
All employers with workers who have a reasonably anticipated risk for BBP exposure must provide an ECP. This plan is a workplace-specific document that outlines jobs/tasks with BBP risk, methods of exposure control, and employer and employee administrative responsibilities. It is imperative that all lab personnel working with human derived materials know the location of and be able to access the current year’s ECP. The plan may be found online at http://biosafety.utk.edu/bbp-exposure-control-plan/.
Researchers and lab personnel are among the occupations that are at risk of being exposed to BBPs and will fall under the specific OSHA regulations designed to reduce occupational risk of infection.
BBPs may be present in infectious concentrations in human blood; semen/vaginal secretions; fluid from the spine, joints, lungs and other vascularized body cavities; any body fluid contaminated with blood; any body fluid that you can’t identify; and other potentially infectious materials (OPIM).
OPIMs in clinical or lab settings include: human blood products (serum, plasma, albumin, various factors, etc.); unfixed tissues/organs (other than intact skin of human origin); cell or tissue cultures that may contain BBPs; organ cultures, culture medium or other solutions that may contain BBPs; experimental animals infected with BBPs.
Note: Tissue cultures of concern include primary and immortalized or established cell lines regardless of origin/vendor unless documented to be free of BBPs. Otherwise, risk assessment indicates coverage under the BBP Standard.
Some human body fluids are not considered to be a BBP hazard unless they are visibly contaminated with blood. These include: urine, feces, vomit, sweat, tears, saliva, or nasal secretions. Even so, use universal precautions and wear appropriate personal protective equipment (PPE) when handling these fluids as they may contain other types of pathogenic microbes.
 Needle sticks and percutaneous injuries are the primary ways BBP infections occur. Other ways infections can occur are: 1) through contact with human derived materials through breaks in the skin that may occur if there is a fresh flesh wound; your skin is chapped; or you have acne, eczema, split cuticles, etc.; 2) splashes of blood or OPIM come into contact with mucous membranes around the eyes, nose or mouth; or 3) transmission occurs through sexual intercourse or from the mother to the unborn child.
Needle sticks and percutaneous injuries are the primary ways BBP infections occur. Other ways infections can occur are: 1) through contact with human derived materials through breaks in the skin that may occur if there is a fresh flesh wound; your skin is chapped; or you have acne, eczema, split cuticles, etc.; 2) splashes of blood or OPIM come into contact with mucous membranes around the eyes, nose or mouth; or 3) transmission occurs through sexual intercourse or from the mother to the unborn child.
An important thing to keep in mind when considering the risk of BBPs is that they are not all equally infectious. The risk of infection through a needlestick exposure for HBV is much higher than for HIV or HCV.
This is in part due to the fact that HIV breaks down quickly once it is outside the human body, while HBV is more environmentally stable. Also, HBV-infected blood/body fluids contain significantly more virus particles relative to HIV-infected blood. This is why the Hepatitis B vaccine is so important.
 OSHA requires employers to offer the Hepatitis B vaccine to all at risk employees as follows: 1) those with risk are offered the vaccine at no cost, 2) the vaccine is offered at a convenient time and location, 3) the offer of vaccine must be documented through a waiver/request to be completed by the employee, and 4) if an employee declines the offer, they can request vaccination at a later date if still at-risk.
OSHA requires employers to offer the Hepatitis B vaccine to all at risk employees as follows: 1) those with risk are offered the vaccine at no cost, 2) the vaccine is offered at a convenient time and location, 3) the offer of vaccine must be documented through a waiver/request to be completed by the employee, and 4) if an employee declines the offer, they can request vaccination at a later date if still at-risk.
The vaccine is typically administered in 3 separate doses spanning ~6 months. Once vaccinated and neutralizing antibodies have been confirmed, boosters are generally not required.
While there are minor risks associated with the vaccine (localized pain, swelling, redness; fatigue; and malaise), it is widely considered one of the safest.
Contact the Biosafety Office for more information about the Hepatitis B vaccine if interested.
 The best way to protect yourself from BBP is to practice universal precautions. Universal precautions approximate BSL-2 practices and include the following:
The best way to protect yourself from BBP is to practice universal precautions. Universal precautions approximate BSL-2 practices and include the following:
- Treat all blood and OPIM as if they were known to be infectious.
- If you are aware of a hazard, communicate it to fellow lab personnel.
- Good laboratory and personal hygiene practices. Routine handwashing and disinfection of potentially contaminated surfaces are critical for mitigating risk!
- Clean the surfaces of visible debris before disinfection with a 1:10 bleach solution or with a disinfectant that is EPA-registered for the destruction of HIV and HBV. Refer to the manufacturer’s instructions for proper dilution, contact time, and use of the disinfectant.
- Eliminate or reduce the use of sharps devices if possible.
- Employ engineering controls when available.
- Wear PPE that is appropriate for the task.
- Segregate and treat wastes (disinfection, autoclaving, etc.).
All employers are required to minimize the risk of needlesticks under the authority of the Needlestick Safety and Prevent Act which is part of the Bloodborne Pathogens standard. The Act specified that employers must provide safer sharps devices if available; employees who work with sharps and blood must be involved in device evaluation and selection process; initial and annual sharps evaluations must be completed by anyone using sharps on LIVE HUMANS (e.g. phlebotomy, finger sticks, etc.); and a sharps injury log must be maintained.
Safer sharps resources and evaluation forms can be found in the Exposure Control Plan or on the Biosafety website.
In the event you are exposed or potentially exposed to a Bloodborne pathogen, it is very important to flush the exposed area for 15 minutes, report the exposure to your supervisor, and seek prompt medical care. It is important because it:
- Allows accurate evaluation of exposure risk by a medical professional;
- Increases the chance of identifying and testing the source of blood/OPIM; and
- Provides lead time to administer treatments that can reduce the chance of infection (if high risk event) – antiretroviral therapies for HIV are most effective if started within 2-4 hours of exposure, and it is important to administer HBV immunoglobulin or vaccine booster.
It is important to take any information that you may have regarding the materials to which you were exposed. This helps to inform the medical risk assessment and course of treatment. Without it, physicians are likely to take the most conservative approach and start antiviral therapies (some of which can have serious side effects).
For all infectious biological material and human derived material exposures, paid staff must report exposure to CorVel Corp. at 1-866-245-8588 to obtain a claim number per UT Risk Management procedures (this step can be concurrent with emergency reporting). Follow CorVel instructions for medical follow-up with health care provider. Paid employees must complete the Worker’s Compensation forms as soon as possible. Forms are to be remitted to the Risk Management Office. For additional information, see http://riskmanagement.tennessee.edu or contact (865) 974-5409.
Unpaid students may report to UT Student Health Services (865-974-3135) or their primary care physician. Unpaid volunteers may report to the health care provider of their choice. Individuals not listed on the UT payroll may be personally responsible for medical costs. Unpaid employees must complete the Incident Report Form found at http://riskmanagement.tennessee.edu or contact (865) 974-5409.
All personnel experiencing an exposure to potentially infectious materials must also complete the Biosafety Incident Report form and submit to the Biosafety Office. The form may be found at https://biosafety.utk.edu/emergency-response/, or by contacting EHS Lab Safety Services at (865) 974-5084.