Transportation of biological agents out of the lab requires lab personnel to take precautions to ensure that lost samples and/or spills in public areas do not occur.

- Use primary containers with lids or tight fitting stoppers to prevent spills.
- When transporting multiple tubes, place in a rack or other type of holder that will keep them from shifting or tipping during transport.
- Consider the use of a leak-resistant secondary container with a lid to contain any leaks or spills while moving through hallways.
- Use a cart with secondary containment to transport large volumes of materials or when moving from floor to floor.
- Avoid contaminating common contact surfaces or non-lab areas. If wearing gloves is necessary, use one glove to hold materials while using a clean, ungloved hand for the manipulation of door knobs, elevator buttons, etc.

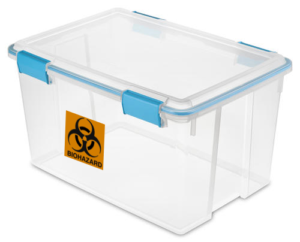
- Follow the guidelines outlined above. Additionally:
Secondary containers are required and must be:
- Leak proof
- Lidded, with a lid that latches into place
- Clearly labeled on the outer surface of secondary container with contact information and a brief description of contents
- Labeled with biohazard symbol if transporting infectious agents or human materials
- Keep biohazardous agents secure
- Place containers in storage areas of vehicles
- Situate containers to prevent shifting during transit
Note: Do not take biological hazards on UT buses or other public transit

Shipment of biological agents by courier (i.e., FedEx or UPS) is permissible in most cases. However, commercial shipping is subject to federal and international regulations, and certain packaging and labeling guidelines apply.
- Non-regulated items include:

- Low risk (RG1) or non-infectious organisms
- Heat-killed or otherwise-inactivated samples
- Patient samples from healthy individuals for routine testing
- Regulated items include:
- Cultures of infectious agents and/or biological toxins
- Human or animal specimens that likely harbor infectious agents
- Genetically modified organisms (in some cases)
- Any materials packaged on dry ice
- Contact the Biosafety Office for assistance in determining the requirements that will apply.
- You cannot submit biohazards for commercial transport unless you have been trained and certified. Training is available from the Biosafety Office for labs that regularly ship materials.
In some cases, transport and/or receipt of biological materials may require permits from U.S. regulatory agencies:
- CDC: Import of infectious agents or vectors affecting humans
- USDA APHIS: Import and interstate movement of livestock/poultry pathogens, animal blood/tissues, plant pests, plant pathogens, & noxious weeds; field release trials for plant pathogens or genetically modified plants and/or plant pathogens
- US FWS: Import/export of biological materials obtained from wildlife
- US DOC: Export of potentially infectious agents affecting humans, animals, or plants from the U.S.
WARNING #1: Biological materials permits may take several weeks to months to obtain. Do not wait until the project is imminent to begin the process of obtaining a permit. Federal agencies will not make exceptions for your needs.
WARNING #2: Do not attempt to circumvent shipping or permit requirements by transporting undeclared biological materials in your carry-on or checked luggage while flying. This may result in substantial fines and/or incarceration.




